

Some of the water molecules have enough kinetic energy to escape the intermolecular forces that are “holding” them in the liquid phase.What happens to a glass of water that is left sitting on a table for an extended period of time?.Calculate the amount of heat (in kJ) needed to heat 150.0 g of C6H6 from 22.0oC to 90.0oC. Its heat of fusion is 9.90 kJ/mole while its heat of vaporization is 30.77 kJ/mol. The specific heat of benzene is 1.516 J/gK in the solid phase, 1.726 J/gK in the liquid phase, and 1.055 J/gK in the gas phase. Phase Changes & Heating Curves Example: Benzene (C6H6) has a boiling point of 80.1 oC and a melting point of 5.5oC. You should be able to handle both types of problems.What would you do differently if the problem asked to calculate the enthalpy change to convert 12.0 g of steam at 115oC to ice at -15oC?.For water, DHfus = 6.01 kJ/mol and DHvap = 40.67 kJ/mol.

The specific heats of ice, water, and steam are 2.09J/g-K, 4.18 J/g-K, and 1.84 J/g-K, respectively. Phase Changes & Heating Curves Example: Calculate the enthalpy change associated with converting 12.0 g of ice at -15oC to steam (water vapor) at 115oC under a constant pressure of one atmosphere. To calculate the amount of heat needed during a phase change (temperature is constant).To calculate the amount of heat needed when the temperature changes (same physical state).The amount of heat needed to covert a solid below its melting point to a gas above its boiling point is found by calculating the amount of heat needed for each step: qtotal = q(Ti MP) + q(sl) + q(MPBP) + q(lg) + q(BPTf).A general heating or cooling curve for a solid below the MP being converted to a gas above the boiling point:.(g, Tf) Cgas DHvap (l, BP) (g, BP) Cliq DHfus (s, MP) (l, MP) Csolid (s, Ti) Phase Changes & Heating Curves The temperature does not increase until the phase change is complete.During a phase change the temperature of the substance is constant.The heat added to the system at the melting point and boiling point goes into pulling the molecules further apart from each other (i.e.At the boiling point, liquid and gas phases are in equilibrium.At the melting point, solid and liquid phases are in equilibrium.
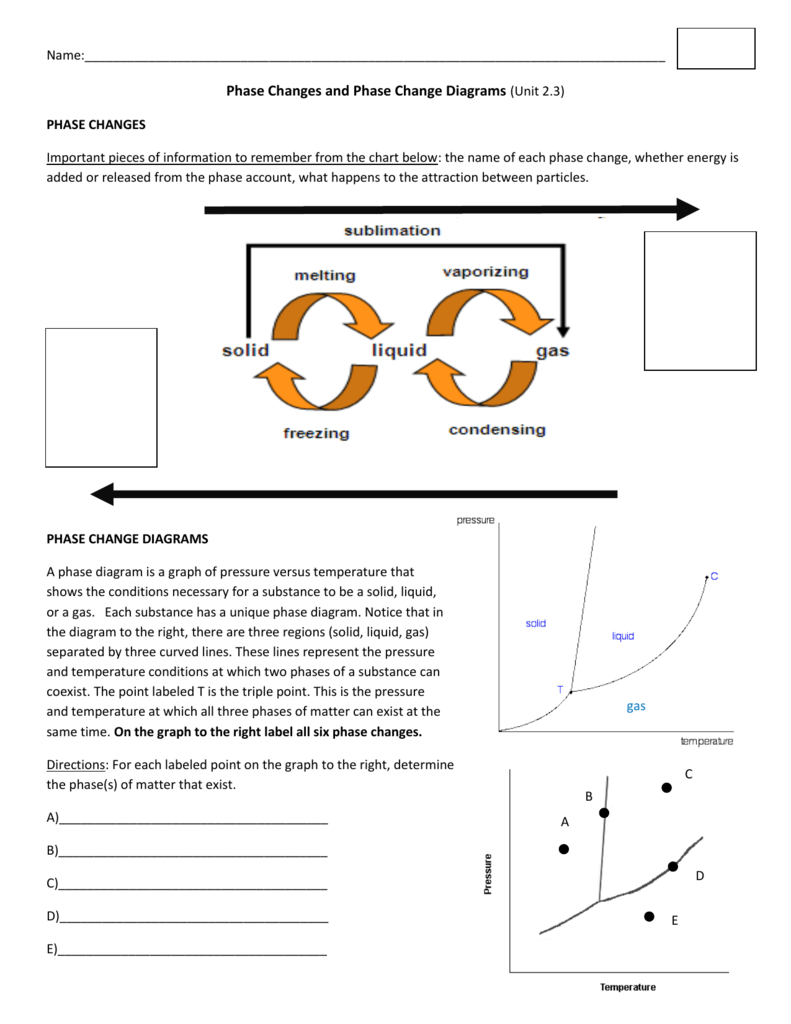
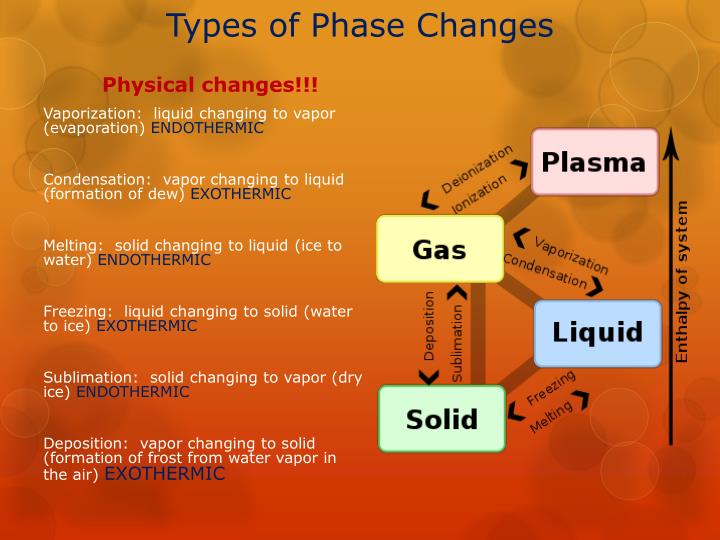
A change in the physical state of a substance.When matter is converted from one physical state to another, a phase change occurs.Matter exists in three physical states:.


 0 kommentar(er)
0 kommentar(er)
